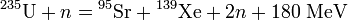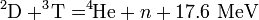Nuclear weapon design

The first nuclear weapons, though large, cumbersome and inefficient, provided the basic design building blocks of all future weapons. Here the Gadget device is prepared for the first nuclear test: Trinity.
Nuclear weapon designs are physical, chemical, and engineering arrangements that cause the physics package[1] of a nuclear weapon to detonate. There are three basic design types. In all three, the explosive energy is derived primarily from nuclear fission, not fusion.
- Pure fission weapons were the first nuclear weapons built and the only type ever used in warfare. The active material is fissile uranium (U-235) or plutonium (Pu-239), explosively assembled into a chain-reacting critical mass by one of two methods:
- Gun assembly, in which one piece of fissile uranium is fired at a fissile uranium target at the end of the weapon, similar to firing a bullet down a gun barrel (plutonium can be used in this design, but it has proven to be impractical), or
- Implosion, in which a fissile mass of either material (U-235, Pu-239, or a combination) is surrounded by high explosives that compress the mass, resulting in criticality.
- Fusion-boosted fission weapons improve on the implosion design. The high temperature and pressure environment at the center of an exploding fission weapon compresses and heats a mixture of tritium and deuterium gas (heavy isotopes of hydrogen). The hydrogen fuses to form helium and free neutrons. The energy release from fusion reactions is relatively negligible, but each neutron starts a new fission chain reaction, greatly reducing the amount of fissile material that would otherwise be wasted. Boosting can more than double the weapon's fission energy release.
- Two-stage thermonuclear weapons are essentially a daisy chain of fusion-boosted fission weapons, with only two daisies, or stages, in the chain. The second stage, called the "secondary," is imploded by x-ray energy from the first stage, called the "primary." This radiation implosion is much more effective than the high-explosive implosion of the primary. Consequently, the secondary can be many times more powerful than the primary, without being bigger. The secondary could be designed to maximize fusion energy release, but in most designs fusion is employed only to drive or enhance fission, as it is in the primary. More stages could be added, but the result would be a multi-megaton weapon too powerful to be useful. (The United States briefly deployed a three-stage 25-megaton bomb, the B41, starting in 1961. Also in 1961, the Soviet Union tested, but did not deploy, a three-stage 50-megaton device, Tsar Bomba.)
Pure fission weapons historically have been the first type to be built by a nation state. Large industrial states with well-developed nuclear arsenals have two-stage thermonuclear weapons, which are the most compact, scalable, and cost effective option once the necessary industrial infrastructure is built.
All innovations in nuclear weapon design originated in the United States;[2] the following descriptions feature U.S. designs.
In early news accounts, pure fission weapons were called atomic bombs or A-bombs, a misnomer since the energy comes only from the nucleus of the atom. Weapons involving fusion were called hydrogen bombs or H-bombs, also a misnomer since their destructive energy comes mostly from fission. Insiders favored the terms nuclear and thermonuclear, respectively.
The term thermonuclear refers to the high temperatures required to initiate fusion. It ignores the equally important factor of pressure, which was considered secret at the time the term became current. Many nuclear weapon terms are similarly inaccurate because of their origin in a classified environment. Some are nonsense code words such as "alarm clock" (see below).
Contents |
Nuclear reactions
Nuclear fission splits the heaviest of atoms to form lighter atoms. Nuclear fusion bonds together the lightest atoms to form heavier atoms. Both reactions generate roughly a million times more energy than comparable chemical reactions, making nuclear bombs a million times more powerful than non-nuclear bombs, which a French patent[3] revendicated in May 1939.
In some ways, fission and fusion are opposite and complementary reactions, but the particulars are unique for each. To understand how nuclear weapons are designed, it is useful to know the important similarities and differences between fission and fusion. The following explanation uses rounded numbers and approximations.[4]
Fission
When a free neutron hits the nucleus of a fissionable atom like uranium-235 ( 235U), the uranium splits into two smaller atoms called fission fragments, plus more neutrons. Fission can be self-sustaining because it produces more neutrons of the speed required to cause new fissions.
The uranium atom can split any one of dozens of different ways, as long as the atomic weights add up to 236 (uranium plus the extra neutron). The following equation shows one possible split, namely into strontium-95 ( 95Sr), xenon-139 ( 139Xe), and two neutrons (n), plus energy:[5]
The immediate energy release per atom is 180 million electron volts (MeV), i.e. 74 TJ/kg, of which 90% is kinetic energy (or motion) of the fission fragments, flying away from each other mutually repelled by the positive charge of their protons (38 for strontium, 54 for xenon). Thus their initial kinetic energy is 67 TJ/kg, hence their initial speed is 12,000 kilometers per second, but their high electric charge causes many inelastic collisions with nearby nuclei. The fragments remain trapped inside the bomb's uranium pit until their motion is converted into x-ray heat, a process which takes about a millionth of a second (a microsecond).
This x-ray energy produces the blast and fire which are the purpose of a nuclear explosion.
After the fission products slow down, they remain radioactive. Being new elements with too many neutrons, they eventually become stable by means of beta decay, converting neutrons into protons by throwing off electrons and gamma rays. Each fission product nucleus decays between one and six times, average three times, producing radioactive elements with half-lives up to 200,000 years.[6] In reactors, these products are the nuclear waste in spent fuel. In bombs, they become radioactive fallout, both local and global.
Meanwhile, inside the exploding bomb, the free neutrons released by fission strike nearby U-235 nuclei causing them to fission in an exponentially growing chain reaction (1, 2, 4, 8, 16, etc.). Starting from one, the number of fissions can theoretically double a hundred times in a microsecond, which could consume all uranium up to hundreds of tons by the hundredth link in the chain. In practice, bombs do not contain that much uranium, and, anyway, just a few kilograms undergo fission before the uranium blows itself apart.
Holding an exploding bomb together is the greatest challenge of fission weapon design. The heat of fission rapidly expands the uranium pit, spreading apart the target nuclei and making space for the neutrons to escape without being captured. The chain reaction stops.
Materials which can sustain a chain reaction are called fissile. The two fissile materials used in nuclear weapons are: U-235, also known as highly enriched uranium (HEU), oralloy (Oy) meaning Oak Ridge Alloy, or 25 (the last digits of the atomic number, which is 92 for uranium, and the atomic weight, here 235, respectively); and Pu-239, also known as plutonium, or 49 (from 94 and 239).
Uranium's most common isotope, U-238, is fissionable but not fissile (meaning that it cannot sustain a chain reaction by itself but can be made to fission, specifically by neutrons from a fusion reaction). Its aliases include natural or unenriched uranium, depleted uranium (DU), tubealloy (Tu), and 28. It cannot sustain a chain reaction, because its own fission neutrons are not powerful enough to cause more U-238 fission. However, the neutrons released by fusion will fission U-238. This reaction produces most of the energy in a typical two-stage thermonuclear weapon.
Fusion
Fusion cannot be self-sustaining because it does not produce the heat and pressure necessary for more fusion. It produces neutrons which run away with the energy. In weapons, the most important fusion reaction is called the D-T reaction. Using the heat and pressure of fission, hydrogen-2, or deuterium ( 2D), fuses with hydrogen-3, or tritium ( 3T), to form helium-4 ( 4He) plus one neutron (n) and energy:[7]
Notice that the total energy output, 17.6 MeV, is one tenth of that with fission, but the ingredients are only one-fiftieth as massive, so the energy output per kilo is greater. However, in this fusion reaction 80% of the energy, or 14 MeV, is in the motion of the neutron which, having no electric charge and being almost as massive as the hydrogen nuclei that created it, can escape the scene without leaving its energy behind to help sustain the reaction – or to generate x-rays for blast and fire.
The only practical way to capture most of the fusion energy is to trap the neutrons inside a massive bottle of heavy material such as lead, uranium, or plutonium. If the 14 MeV neutron is captured by uranium (either type: 235 or 238) or plutonium, the result is fission and the release of 180 MeV of fission energy, which will produce the heat and pressure necessary to sustain fusion, in addition to multiplying the energy output tenfold.
Fission is thus necessary to start fusion, to sustain fusion, and to optimize the extraction of useful energy from fusion (by making more fission). In the case of a neutron bomb, see below, the last-mentioned does not apply since the escape of neutrons is the objective.
Tritium production
A third important nuclear reaction is the one that creates tritium, essential to the type of fusion used in weapons and, incidentally, the most expensive ingredient in any nuclear weapon. Tritium, or hydrogen-3, is made by bombarding lithium-6 ( 6Li) with a neutron (n) to produce helium-4 ( 4He) plus tritium ( 3T) and energy:[7]
A nuclear reactor is necessary to provide the neutrons. The industrial-scale conversion of lithium-6 to tritium is very similar to the conversion of uranium-238 into plutonium-239. In both cases the feed material is placed inside a nuclear reactor and removed for processing after a period of time. In the 1950s, when reactor capacity was limited, for the production of every atom of tritium the production of an atom of plutonium had to be dispensed with.
The fission of one plutonium atom releases ten times more total energy than the fusion of one tritium atom, and it generates fifty times more blast and fire. For this reason, tritium is included in nuclear weapon components only when it causes more fission than its production sacrifices, namely in the case of fusion-boosted fission.
However, an exploding nuclear bomb is a nuclear reactor. The above reaction can take place simultaneously throughout the secondary of a two-stage thermonuclear weapon, producing tritium in place as the device explodes.
Of the three basic types of nuclear weapon, the first, pure fission, uses the first of the three nuclear reactions above. The second, fusion-boosted fission, uses the first two. The third, two-stage thermonuclear, uses all three.
References
Specific
- ^ The physics package is the nuclear explosive module inside the bomb casing, missile warhead, or artillery shell, etc., which delivers the weapon to its target. While photographs of weapon casings are common, photographs of the physics package are quite rare, even for the oldest and crudest nuclear weapons. For a photograph of a modern physics package see W80.
- ^ The United States and the Soviet Union were the only nations to build large nuclear arsenals with every possible type of nuclear weapon. The U.S. had a four-year head start and was the first to produce fissile material and fission weapons, all in 1945. The only Soviet claim for a design first was the Joe 4 detonation on August 12, 1953, said to be the first deliverable hydrogen bomb. However, as Herbert York first revealed in The Advisors: Oppenheimer, Teller and the Superbomb (W.H. Freeman, 1976), it was not a true hydrogen bomb (it was a boosted fission weapon of the Sloika/Alarm Clock type, not a two-stage thermonuclear). Soviet dates for the essential elements of warhead miniaturization – boosted, hollow-pit, two-point, air lens primaries – are not available in the open literature, but the larger size of Soviet ballistic missiles is often explained as evidence of an initial Soviet difficulty in miniaturizing warheads.
- ^ http://v3.espacenet.com/maximizedOriginalDocument?KC=A&date=19510116&flavour=plainPage&NR=971324A&locale=fr_FR&CC=FR&FT=D
- ^ The main source for this section is Samuel Glasstone and Philip Dolan, The Effects of Nuclear Weapons, Third Edition, 1977, U.S. Dept of Defense and U.S. Dept of Energy (see links in General References, below), with the same information in more detail in Samuel Glasstone, Sourcebook on Atomic Energy, Third Edition, 1979, U.S. Atomic Energy Commission, Krieger Publishing.
- ^ Glasstone and Dolan, Effects, p. 12.
- ^ Glasstone, Sourcebook, p. 503.
- ^ a b Glasstone and Dolan, Effects, p. 21.
- ^ Glasstone and Dolan, Effects, p. 12-13. When one pound (454 g) of U-235 undergoes complete fission, the yield is 8 kilotons. The 13-to-16-kiloton yield of the Little Boy bomb was therefore produced by the fission no more than two pounds (907 g) of U-235, out of the 141 pounds (64 kg) in the pit. The remaining 139 pounds (63 kg), 98.5% of the total, contributed nothing to the energy yield.
- ^ Compere, A.L., and Griffith, W.L. 1991. "The U.S. Calutron Program for Uranium Enrichment: History,. Technology, Operations, and Production. Report," ORNL-5928, as cited in John Coster-Mullen, "Atom Bombs: The Top Secret Inside Story of Little Boy and Fat Man," 2003, footnote 28, p. 18. The total wartime output of Oralloy produced at Oak Ridge by July 28, 1945 was 165 pounds (74.68 kg). Of this amount, 84% was scattered over Hiroshima (see previous footnote).
- ^ "Restricted Data Declassification Decisions from 1945 until Present" - "Fact that plutonium and uranium may be bonded to each other in unspecified pits or weapons."
- ^ "Restricted Data Declassification Decisions from 1946 until Present"
- ^ Fissionable Materials section of the Nuclear Weapons FAQ, Carey Sublette, accessed Sept 23, 2006
- ^ All information on nuclear weapon tests comes from Chuck Hansen, The Swords of Armageddon: U.S. Nuclear Weapons Development since 1945, October 1995, Chucklea Productions, Volume VIII, p. 154, Table A-1, "U.S. Nuclear Detonations and Tests, 1945-1962."
- ^ Nuclear Weapons FAQ: 4.1.6.3 Hybrid Assembly Techniques, accessed December 1, 2007. Drawing adapted from the same source.
- ^ Nuclear Weapons FAQ: 4.1.6.2.2.4 Cylindrical and Planar Shock Techniques, accessed December 1, 2007.
- ^ "Restricted Data Declassification Decisions from 1946 until Present", Section V.B.2.k "The fact of use in high explosive assembled (HEA) weapons of spherical shells of fissile materials, sealed pits; air and ring HE lenses," declassified November 1972.
- ^ Until a reliable design was worked out in the early 1950s, the hydrogen bomb (public name) was called the superbomb by insiders. After that, insiders used a more descriptive name: two-stage thermonuclear. Two examples. From Herb York, The Advisors, 1976, "This book is about . . . the development of the H-bomb, or the superbomb as it was then called." p. ix, and "The rapid and successful development of the superbomb (or super as it came to be called) . . ." p. 5. From National Public Radio Talk of the Nation, November 8, 2005, Siegfried Hecker of Los Alamos, "the hydrogen bomb – that is, a two-stage thermonuclear device, as we referred to it – is indeed the principal part of the US arsenal, as it is of the Russian arsenal."
General
- Cohen, Sam, The Truth About the Neutron Bomb: The Inventor of the Bomb Speaks Out, William Morrow & Co., 1983
- Glasstone, Samuel and Dolan, Philip J., The Effects of Nuclear Weapons (third edition) (hosted at the Trinity Atomic Web Site), U.S. Government Printing Office, 1977. PDF Version
- Grace, S. Charles, Nuclear Weapons: Principles, Effects and Survivability (Land Warfare: Brassey's New Battlefield Weapons Systems and Technology, vol 10)
- Hansen, Chuck, The Swords of Armageddon: U.S. Nuclear Weapons Development since 1945, October 1995, Chucklea Productions, eight volumes (CD-ROM), two thousand pages.
- The Effects of Nuclear War, Office of Technology Assessment (May 1979).
- Rhodes, Richard. The Making of the Atomic Bomb. Simon and Schuster, New York, (1986 ISBN 0-684-81378-5)
- Rhodes, Richard. Dark Sun: The Making of the Hydrogen Bomb. Simon and Schuster, New York, (1995 ISBN 0-684-82414-0)
- Smyth, Henry DeWolf, Atomic Energy for Military Purposes, Princeton University Press, 1945. (see: Smyth Report)
External links
| Wikimedia Commons has media related to: Nuclear weapon design |
- Carey Sublette's Nuclear Weapon Archive is a reliable source of information and has links to other sources.
- Nuclear Weapons Frequently Asked Questions: Section 4.0 Engineering and Design of Nuclear Weapons
- The Federation of American Scientists provides solid information on weapons of mass destruction, including nuclear weapons and their effects
- Globalsecurity.org provides a well-written primer in nuclear weapons design concepts (site navigation on righthand side).
- More information on the design of two-stage fusion bombs
- Militarily Critical Technologies List (MCTL) from the US Government's Defense Technical Information Center
- "Restricted Data Declassification Decisions from 1946 until Present", Department of Energy report series published from 1994 until January 2001 which lists all known declassification actions and their dates. Hosted by Federation of American Scientists.
- The Holocaust Bomb: A Question of Time is an update of the 1979 court case USA v. The Progressive, with links to supporting documents on nuclear weapon design.
- Annotated bibliogrphy on nuclear weapons design from the Alsos Digital Library for Nuclear Issues